特别是高浓度制剂的mAbs药品更容易受到给药设备功能性和药物与容器相互作用的影响。

药物在一段时间内的稳定性、药效和安全性

一步到位的开发

为改善治疗依从性的可靠给药系统

更高浓度和注射量
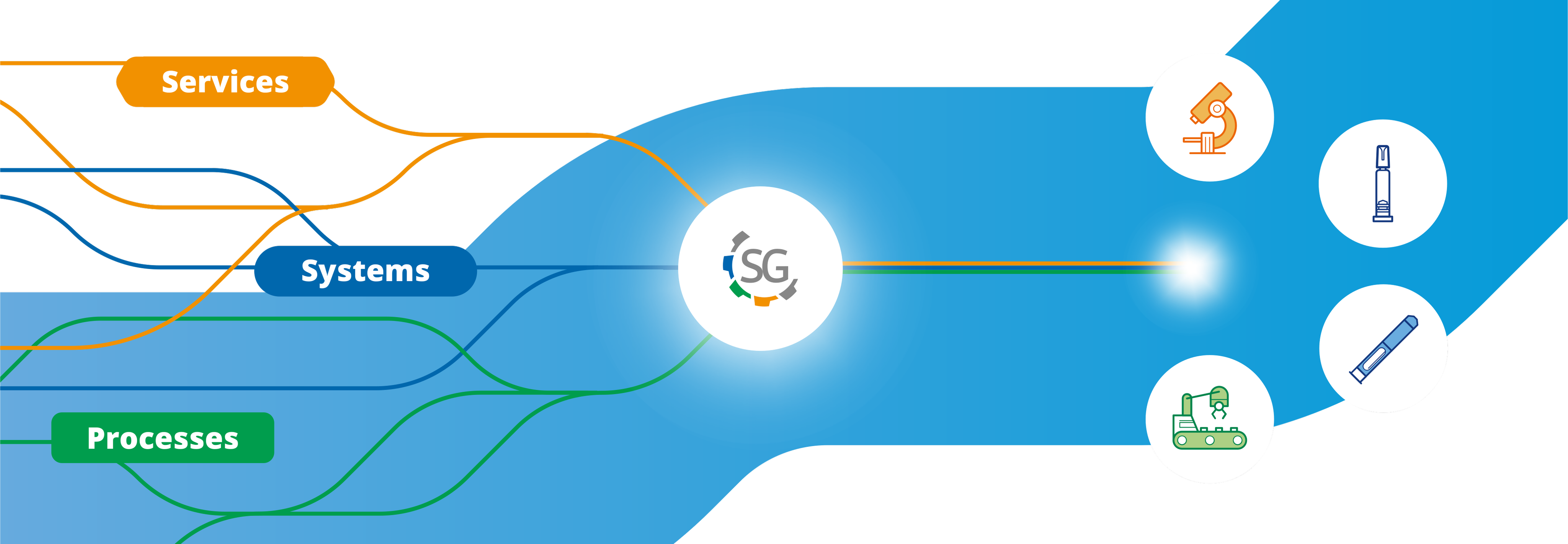
斯蒂瓦那托集团 (Stevanato Group) 能够为mAbs的生产过程提供覆盖整个制药价值链的全面性解决方案。我们独特的市场定位让我们能够成为内包材、设备、机械和分析服务的综合供应商。
Trend and insights
The biologics market is growing enormously. Due to the low stability of biologics, 24% of biologic drugs newly approved by the FDA between 2017 and 2021 are lyophilized.
Challenge
EZ-fill® nested vials are in a suspension frame “nest”, contrary to conventional loading where the vials are in direct contact with the freeze-dryer shelf. The suspended configuration avoids glass-to-glass contact during the filling phase and reduces mechanical stress which could lead to vial breakage but it may raise doubts about the heat transfer mechanism from the shelf to the vials.
Solution
Stevanato Group, in collaboration with the Politecnico di Torino University (POLITO), has conducted a series of tests on freeze-drying pharmaceuticals in nested vials demonstrating that EZ-fill® Vials are high-value solutions for lyo products.
The overall study results, performed on a 3ml ISO vial, not only confirmed the usability of nested vials for lyophilization but, above all, demonstrated their potential as a high-value solution for freeze-dried pharmaceutical products.
Objective
verify thermal properties of the nest during and after freeze-drying process, focusing on the impact on time of freeze-drying cycle and on intra-batch variability
Results
EZ-Fill® Nest & Tub configuration, in comparison to Bulk configuration:
Conclusions