It is estimated that the GLP-1 market will exceed $100 bn by 2030, driven mainly by diabetes and obesity treatment. In the US alone, total GLP-1 users could reach 30 million by 2030 – that’s around 9% of the overall population[1]. At Stevanato Group we believe that today, and for the foreseeable future, auto-injectors and pen injectors will remain at the forefront of GLP-1 delivery. However, altough this potential blockbuster treatment presents considerable opportunities for pharma partners, there are challenges worth considering - from drug and device compatibility to supply chain, given the high demand.
[1] - Source: https://www.jpmorgan.com/insights/global-research/current-events/obesity-drugs
For pharma companies aiming to satisfy the surging demand, the choice of delivery mechanism and delivery partner will have implications for supply chain capacity and resilience. Optimising the route to market for a GLP-1 & Peptides device requires assessment and resolution of risk in areas including drug containment and device specification as well as scaled-up production, assembly, fill and finish, and distribution.
Access to proven technologies, relevant device options, global manufacturing expertise and go-to-market knowledge are, therefore, all integral to supporting drug companies’ commercial strategies with a time-efficient and cost-effective route to market.

Significant demand puts pressure on supply chain
Ready-to-Use (RTU) primary packaging availability is critical to ensure clinical supplies and scale-up

Mitigate risks from development to commercial

Time to market is a key variable to seize the opportunity

Minimize drug product wastage

Fixed vs variable dose - single or multiple injections
To follow patient preferences across geographies, both pens and auto-injectors are viable
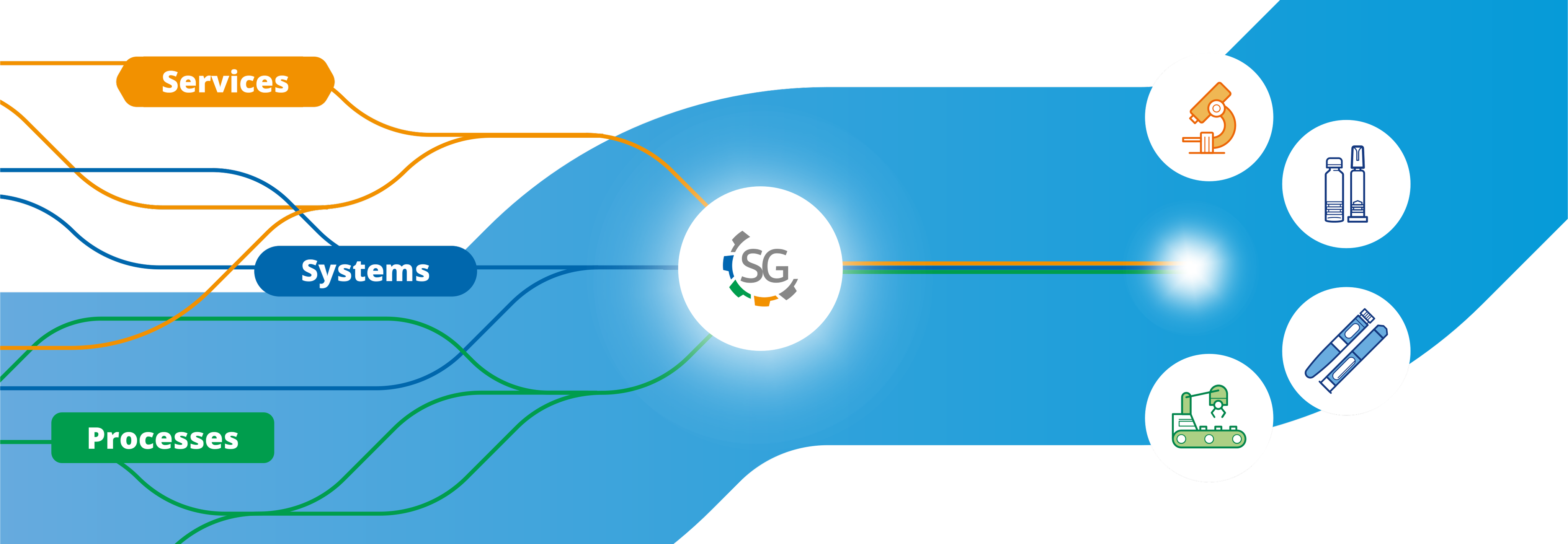
Stevanato Group can serve GLP-1 & Peptides production processes with comprehensive solutions covering the full pharma value chain.
Our unique position in the market enables us to be an integrated provider of primary packaging, devices, machinery, and analytical services