The biologics market is projected to experience a double-digit (+15%) compound annual growth rate (CAGR) until 2027[1]. Stevanato Group offers novel glass drug containment solutions and drug delivery devices suitable for the new generation of drugs, combined with state-of-the-art analytical services, and reliable technologies and manufacturing equipment.
[1] - Source: IQVIA and Stevanato Group internal analysis
mAbs products, for example some highly concentrated formulations, are more susceptible to drug-container interaction and delivery device functionality.

Stability, potency, and safety of drugs over time

Right at the first-time development

Reliable delivery systems to improve therapy adherence

Higher concentration and injection volume
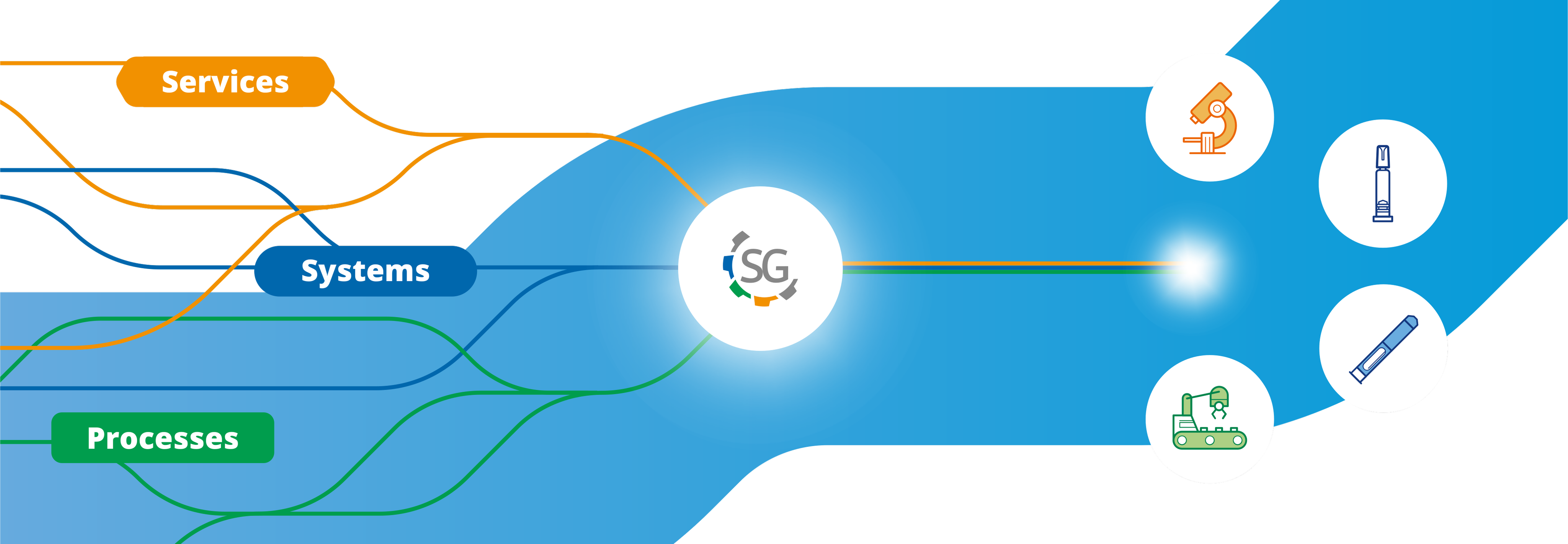
Stevanato Group can serve mAbs production processes with comprehensive solutions covering the full pharma value chain. Our unique position in the market enables us to be an integrated provider of primary packaging, devices, machinery and analytical services.
Trend and insights
The biologics market is growing enormously. Due to the low stability of biologics, 24% of biologic drugs newly approved by the FDA between 2017 and 2021 are lyophilized.
Challenge
EZ-fill® nested vials are in a suspension frame “nest”, contrary to conventional loading where the vials are in direct contact with the freeze-dryer shelf. The suspended configuration avoids glass-to-glass contact during the filling phase and reduces mechanical stress which could lead to vial breakage but it may raise doubts about the heat transfer mechanism from the shelf to the vials.
Solution
Stevanato Group, in collaboration with the Politecnico di Torino University (POLITO), has conducted a series of tests on freeze-drying pharmaceuticals in nested vials demonstrating that EZ-fill® Vials are high-value solutions for lyo products.
The overall study results, performed on a 3ml ISO vial, not only confirmed the usability of nested vials for lyophilization but, above all, demonstrated their potential as a high-value solution for freeze-dried pharmaceutical products.
Objective
verify thermal properties of the nest during and after freeze-drying process, focusing on the impact on time of freeze-drying cycle and on intra-batch variability
Results
EZ-Fill® Nest & Tub configuration, in comparison to Bulk configuration:
Conclusions