Executive Summary
Evaluating the performance of containers on a case-by-case basis is always recommended. But increased assurance about the compatibility of glass containers with cold storage conditions is key for pharmaceutical companies facing development issues with mRNA vaccines. In this increasingly complex application, Stevanato Group glass vials are a proven solution to make life easier for customers.

This study aims to demonstrate experimentally the Mechanical & Container Closure Integrity (CCI) performance of 2R Fina® quality glass vials in multiple conditions.
Mechanical performance was tested with two (2) quantitative techniques, Vertical Compression Test (VT) and Burst Test (BT), measuring the maximum mechanical stress that a single vial can withstand before generating a failure; the configurations tested showed no statistically significant difference before and after conditioning.
CCI was tested with a deterministic technique, Laser-based Headspace-Gas Analysis, measuring the concentration of CO2 gas present in the headspace of the vial. In this study, the analysis was conducted on manually and automatically assembled vials with multiple stopper configurations, at multiple timepoints after storage at room temperature and at -80°C. Seven (7) combinations were tested, each with 30 samples at T0,T6 and T24 months, and all configurations passed, showing that CCI was maintained.
Given that final container closure configuration is process and material dependent, a dedicated study is mandatory to ensure the suitability of the selected configuration for the required application.
Test Approach
Verification of mechanical resistance with Vertical Compression Test & Burst Test
The objective of these tests was to identify and determine the impact (if any) on glass vials’ mechanical resistance to Axial Load and Internal Pressure of storage conditions of -80°C over a period of 7 days. The samples for which VC and BT were evaluated were uncapped 2R vials to evaluate only the impact on the glass container itself.
VC and BT were performed on empty vials with and without Deep Freezing conditioning to verify any differences in behaviour caused by the conditioning itself.
VC and BT tests were performed using a Zwick Roell Z050 universal testing machine

Figure 1: a) Example of sample before Vertical Compression. b) Example of sample after Vertical Compression.

Figure 2: a) Example of sample before Burst Test. b) Example of sample after Burst Test.
The categories tested were 2R vials according to ISO 8362-1, with two different configurations:
2R Fina®: 2R vials without blowback and with standard bottom design, manufactured to Fina® Quality level.
2R Fina®: 2R vials with European blowback and with bottom design optimized for lyophilization, manufactured to Fina® Quality level.

Figure 3: Climatic chamber with 2R vials inside for DF cycle.
To study the possible influence of the Filling Volume (FV) on the mechanical resistance of vials during the freezing process, a preliminary feasibility study was performed to assess an “optimal” FV that does not cause any cracks or breakage of vials after the DF cycle.
Since no damaged vials were noticed, even after an FV of 3,0 ml, this value was chosen as an “optimal” FV. FV 3,0 ml is the FV used for the DF cycle to obtain the “post-freezing” samples.
Verification of CCI with Headspace Gas Test
The objective of this test was to determine CCI during stability at 25°C/60% RH and -80°C at three different time points (T0, T6 and T24 months) over a storage period of 24 months.
The samples for which CCI was evaluated were 2R vials with different cap methods: stoppered with aluminum crimped cap and capped with a press-fit cap.
CCI testing was conducted using Headspace Gas Analysis, based on the measurement of the headspace carbon dioxide content in the samples after exposure to a carbon dioxide enriched environment at the target temperatures.
The categories tested were 2R vials according to ISO 8362-1, with only one configuration:
2R Fina®: 2R vials without blowback and with standard bottom design, manufactured to Fina® Quality level.
Test results and conclusions
Mechanical performances of Vertical Compression and Burst Test
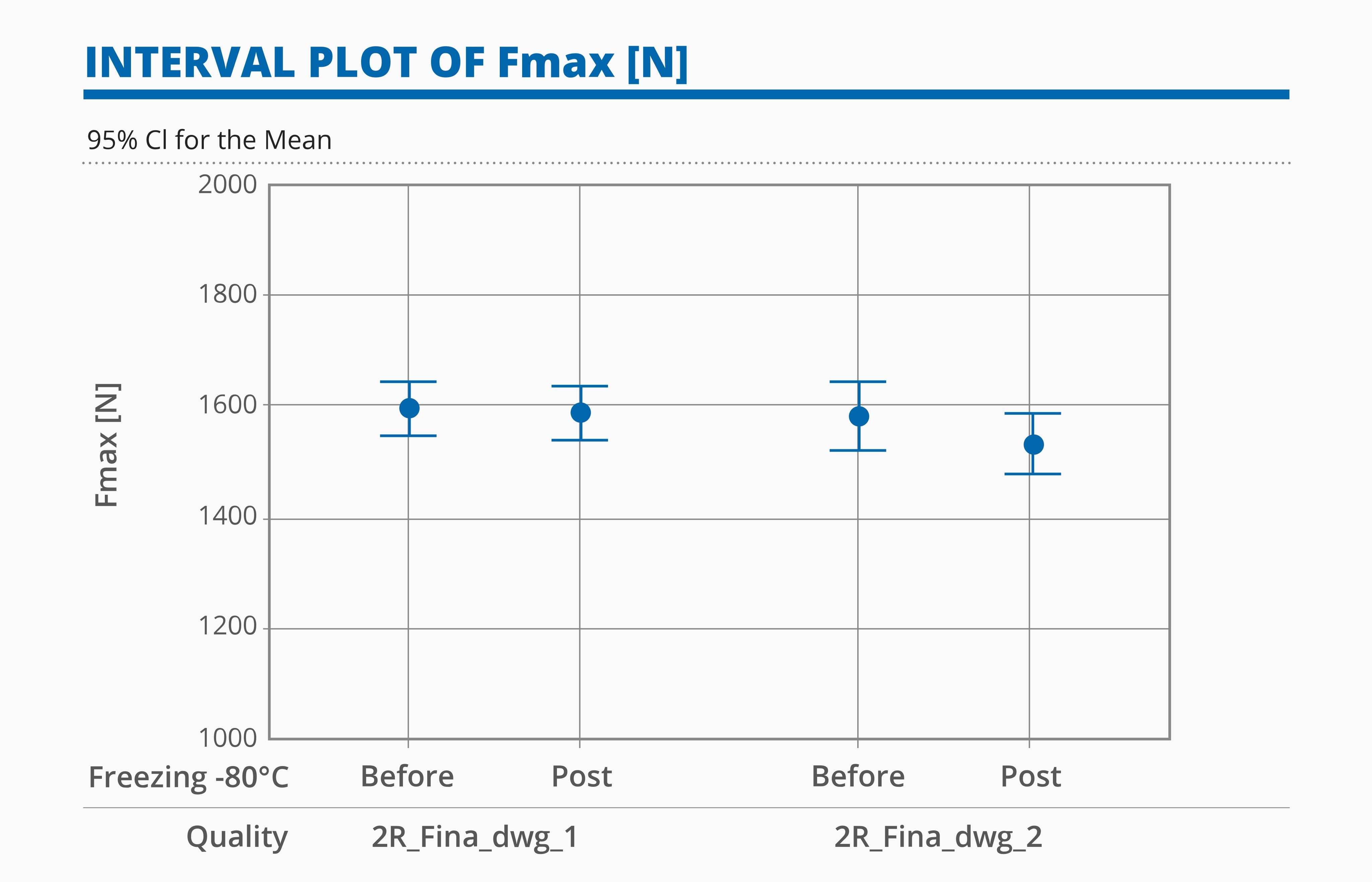
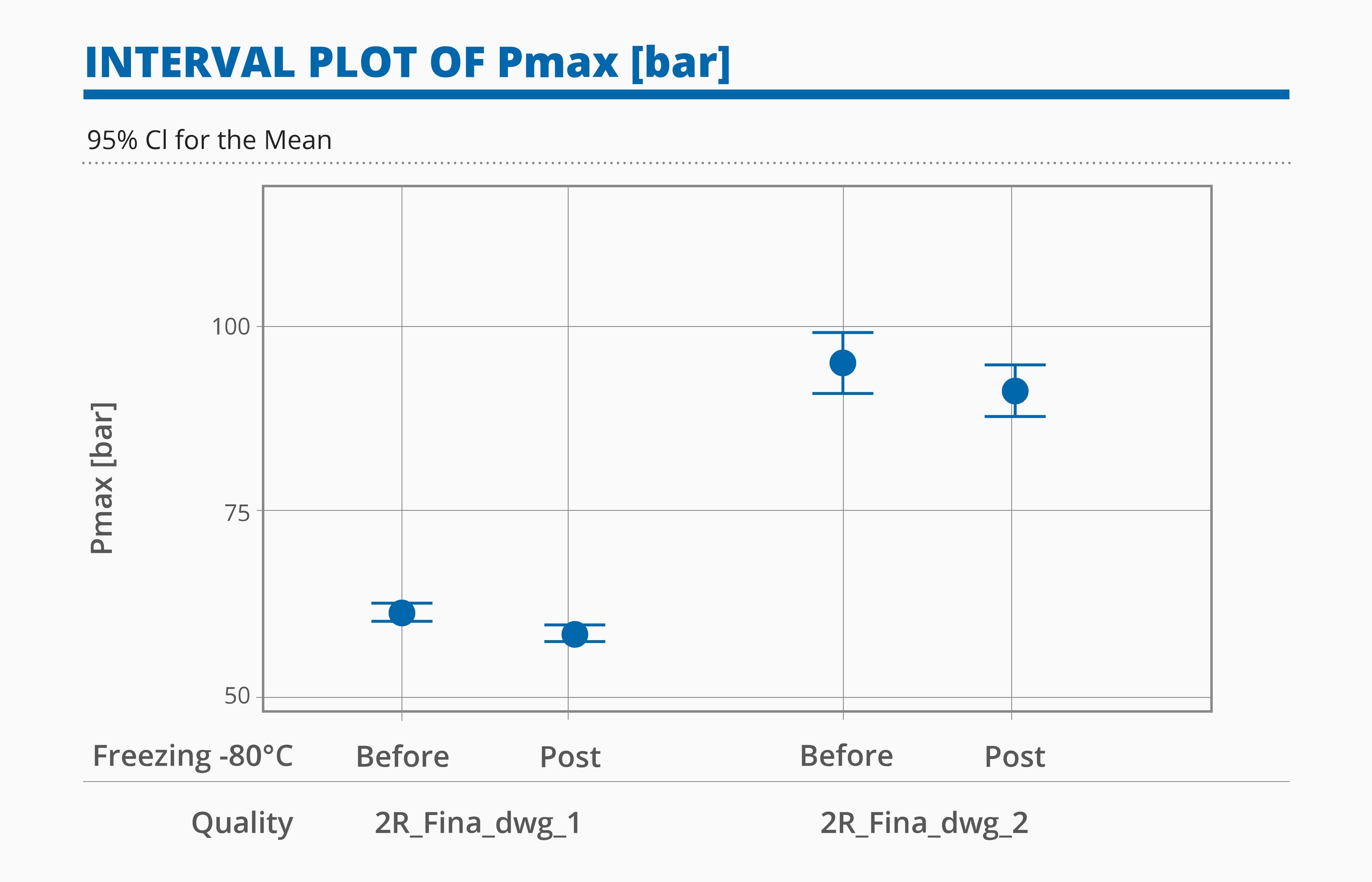
The results of the two categories tested with VT and BT are summarized below:
The following effects can be asserted:
- Both categories “2R_Fina_dwg1” and “2R_Fina_dwg2” showed little or no reduction of the average maximum force that generates breakages after the conditioning and no significant difference in mechanical performance post DF.
- No impact of the mouth geometry (no blowback or European blowback) or the bottom geometry (standard bottom and bottom optimized for lyophilization) on Vertical compression results
- Vials with European blowback and bottom optimized for lyophilization showed better mechanical properties for the BT compared with vials with no blowback and standard bottom design.
- The categories tested showed no statistical difference after being conditioned at -80°C.
CCIT - Headspace Gas Analysis (CO2)
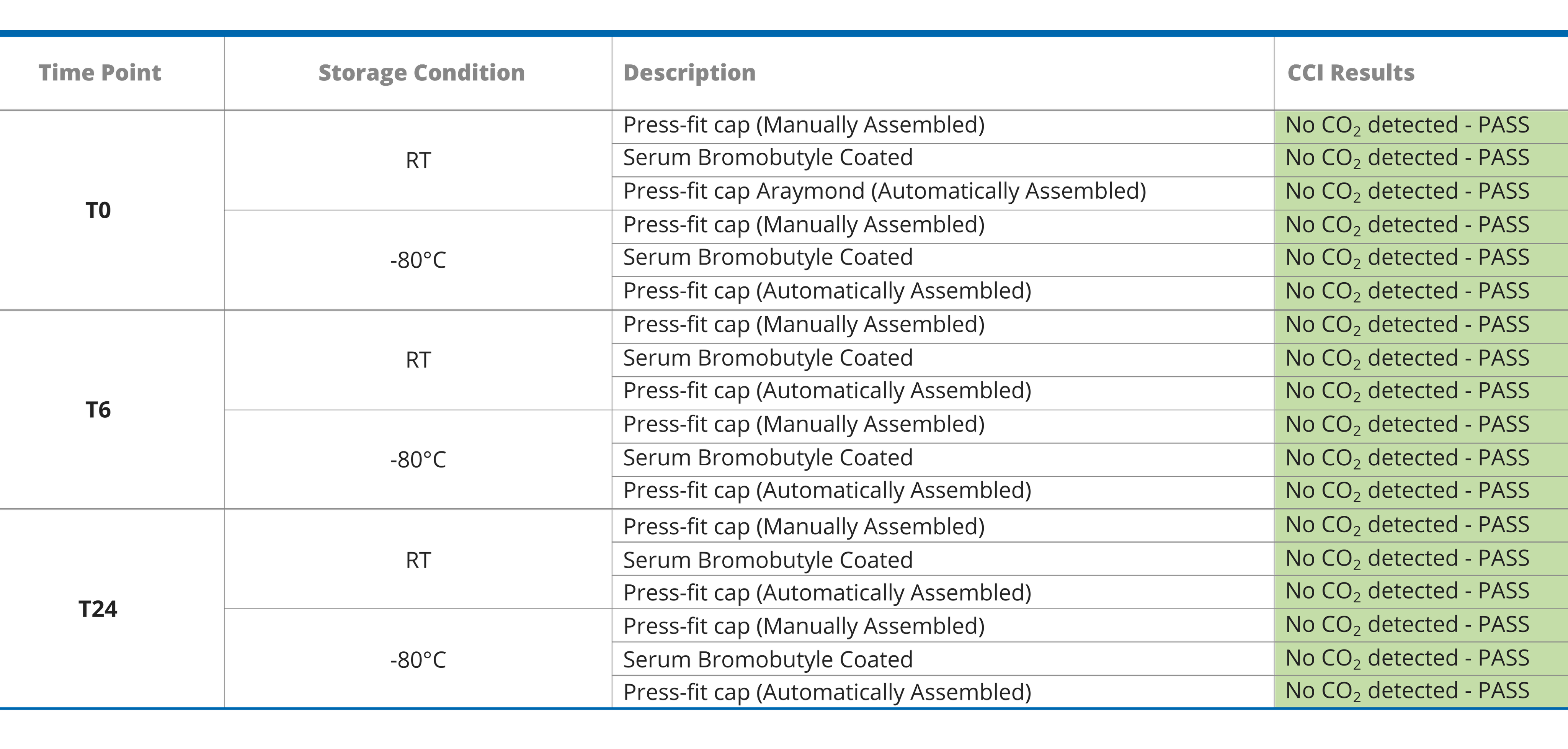
In Table, the HGA CCI test results are summarized for each tested configuration under every condition.
The following effects can be asserted:
- All the test samples retained CCI throughout the storage, both at 25°C and -80°C, independently of the stopper and assembling type.
- All the positive controls, both gross and laser-drilled, showed high values of CO2 ingress (data not shown). The method is therefore capable of detecting holes of a size of 5 µm at RT and -80°C.
- No issues with vial breakages were observed with any of the categories tested.
- The categories tested maintained CCI at -80°C and RT, at T0, T6 and T24.
Conclusions
This study analyzed and verified the behavior of 2R Fina® quality glass vials from Stevanato Group after being conditioned at -80°C for a prolonged period of time.
The results of such analysis show that 2R vials can be processed under such conditions without impacting their mechanical performance; moreover, vial geometry, in combination with different closure systems, can maintain CCI for a prolonged period of time under the testing conditions.
Stevanato Group’s 2R Fina® quality glass vials are therefore suitable for sensitive molecules that require deep-cold storage to keep the drug stable until it is used – mRNA and DNA vaccines, for example, as well as viral vector and gene therapies.









